Abstract
Introduction: Immune thrombocytopenia (ITP) is an acquired thrombocytopenia and caused by immune-mediated platelet destruction and impaired platelet production. Corticosteroids are recommended as the standard first-line treatment. As the immunosuppressive therapy,HD-DXM could decrease platelet destruction by regulating T cell immune abnormalities. Thrombopoietin (TPO) is the primary regulator of thrombopoiesis. Several studies have proved that TPO have profound effects on megakaryocyte development and platelet production as well as restore immune tolerance. We suppose the rhTPO and HD-DXM could work synergistically based on these mechanisms. Recently, several pilot studies have examined the efficacy and safety of TPO-RA in treatment-naive adult ITP patients. We present the results of the first prospective,multicenter,randomized,controlled trial on the largest cohort to date comparing the efficacy and safety of HD-DXM plus rhTPO vs HD-DXM as frontline therapy in newly diagnosed adult primary ITP patients.
Methods: Between July 2013 and December 2016, 245 newly-diagnosed, treatment-naive ITP patients aged 18-75 years in 25 separate centers in China were enrolled in the trial (NCT01734044). DXM was administered orally at 40 mg daily for 4 consecutive days to both arms. The 4-day course of dexamethasone was repeated on days 11 to 14 in the case of lack of response. Patients in the experimental arm received subcutaneously rhTPO at a daily dose of 300U/kg concomitantly during the first 14 days. Patients with platelet counts higher than 100×109/L or more than 50×109/L increase of the baseline platelet count could discontinue the rhTPO treatments. Use of rescue treatments such as platelet transfusion and hemostatic agents were allowed at the discretion of the investigator if the platelet count <10×109/L or ≥10×109/L with active bleeding and were recorded. Patients were assessed at day 14. Study visits were scheduled every 1 month until the end of the 6th month or day of relapse. The primary endpoint was the early response rates at day 14 (OR: platelet count ≥30×109/L and at least 2-fold increase of the baseline platelet count and absence of bleeding, CR: platelet count ≥100×109/L). Key secondary endpoints were rates of sustain response and complete response at the end of month 6, duration of response (DOR), bleeding scores, assessments of adverse events (AEs). Any administration of additional ITP specific medication or withdrawn from the study because of no response was regarded as nonresponders.
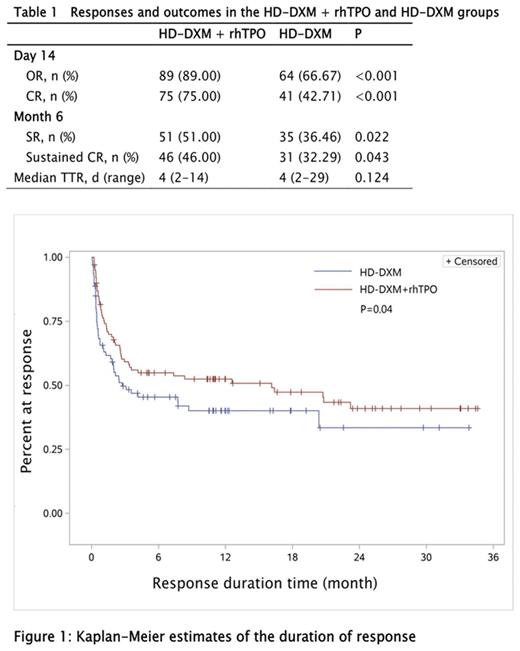
Results: 196 patients were randomly to receive HD-DXM with (n=100) or without (n=96) rhTPO. Demographics and baseline characteristics were balanced between the 2 arms. HD-DXM+ rhTPO resulted in a higher incidence of early OR at day 14 compared with HD-DXM monotherapy (89.0% vs 66.7%, P<0.001, table 1), the CR rate were 75.0% (HD-DXM+ rhTPO) vs 42.7% (HD-DXM) (P<0.001). Sustained OR and CR at the end of the 6th month was also higher in patients treated with HD-DXM+ rhTPO than in those treated with HD-DXM alone (OR: 51.0% vs 36.5%, P=0.022; CR: 46.0% vs 32.3%, P=0.043, table 1). Throughout the follow-up period, overall duration of response was greater in the experimental group, estimated by the Kaplan-Meier analysis (Figure 1, P=0.04). There was no difference between these 2 groups in terms of TTR, bleeding scores and rescue treatments. No statistically significant differences were observed in the incidence of treatment-related AEs between the two groups. Three patients had grade 4 or higher AEs in the HD-DXM+ rhTPO group, including early cerebral hemorrhage (n=2) and cerebral infarction (n=1); none were deemed treatment-related, one patient in the rhTPO group died of early cerebral hemorrhage. No patient was tested positive for neutralizing antibodies against TPO.
Conclusions: Our findings suggest that the addition of rhTPO to HD-DXM is superior to HD-DXM monotherapy in the treatment of newly diagnosed treatment-naive adult ITP patients. Thus, this combination can be a feasible frontline therapy in adult ITP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal